Tc-99m radiopharmaceuticals is the most frequently used medical isotope in nuclear medicine. Japan relies Tc-99m raw material Mo-99 on 100% import. During the past decades, global Mo-99 shortages were not unusual, due to malfunction of nuclear power plants in export countries and other reasons.2)
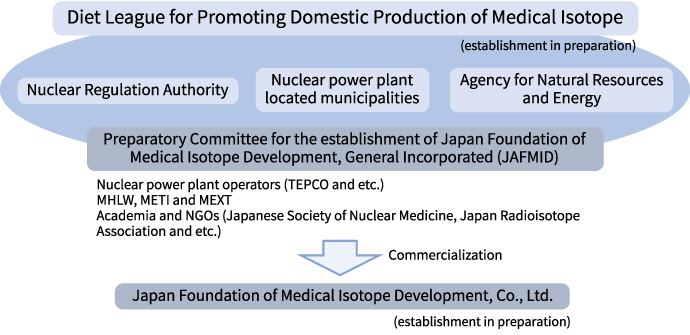
With an aim to addressing this issue, we are herewith establishing a multi-stakeholder platform to drive domestic production of Tc products, by neutron activation of Mo-98 into Mo-99, leveraging existing Boiling Water Reactors and research nuclear power reactors currently in development.
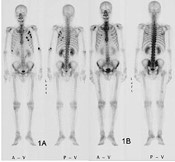
1) Tc products are commonly used in bone, thyroid and other scintigraphy
2) Quoted from Action Plan for stable Tc product Supply in Japan (July 7th, 2011), Public and Private Partnership for Mo-99/Tc-99m Stable Supply (document in Japanese)
Launch of Commission for Commercialization of Mo-99 Production (July 26th, 2011) (document in Japanese)
2) Quoted from Action Plan for stable Tc product Supply in Japan (July 7th, 2011), Public and Private Partnership for Mo-99/Tc-99m Stable Supply (document in Japanese)
Launch of Commission for Commercialization of Mo-99 Production (July 26th, 2011) (document in Japanese)
Technical Advisory Panel
Dr. Jun Hatazawa, M.D., Ph.D 畑澤順
Chairman of the Board, JAFMID
Chairman and Professor, Department of Nuclear Medicine and Tracer Kinetics, Osaka University Graduate School of Medicine
Chairman, PET Molecular Imaging Division, Medical Imaging Center, Osaka University Graduate School of Medicine
Chairman of the Board, Japanese Society of Nuclear Medicine
Dr. Takashi Yamashita, M.D., Ph.D 山下孝
Member of the Board, JAFMID
Senior Board Member, Japan Radioisotope Association, Public Interest Incorporated Foundation
Former Vice-President of The Cancer Institute Hospital of JFCR
Dr. Takeo Morooka, M.D., M.P.H. 諸岡健雄
Founder and Member of the Board, JAFMID
Executive Officer, MSD K.K.
Chairman and CEO, MSD Life Science Foundation, Public Interest Incorporated Foundation
Dr. Takeshi Sasaki, M.D. 佐々木健
Technical Advisor, JAFMID
Director, Medical Professions Division, Health Policy Bureau, Ministry of Health, Labour and Welfare
Dr. Anri Inaki, M.D., Ph.D 稲木杏吏
Technical Advisor, JAFMID
Deputy Director, Regional Medical Care Planning Division, Health Policy Bureau, MHLW
Research Fellow, Department of Nuclear Medicine and Biotracker Medicine, Kanazawa University
